




Resonant Transmutation of Nitrogen in Lung Tissue
Carbon Monoxide Poisoning by Transmutation of Nitrogen in the Lungs
by Alex Putney for Human-Resonance.org
January 22, 2013 -- Updated April 17, 2017
The following is an interesting excerpt from C. Louis Kervran's 'Biological Transmutations' (Crosby Lockwood, 1972), translated from the original French by Crosby Lockwood and revised and edited by Herbert and Elizabeth Rosenauer, pages 18-21:
In 1935... a case of fatal poisoning of a welder had occurred... [yet] I could find no evidence to show where the carbon monoxide originated... It was only in 1955 that it dawned upon me what had happened. In that year... three welders using blow-pipes had died in a period of several months... from carboxyhaemoglobinaemia (carbon monoxide poisoning)... It was decided... to take blood samples from fellow workers even though the men were apparently in good health. The samples showed that those doing the same work as the victims were seriously afflicted with chronic carboxyhaemoglobinaemia, some to a degree approaching that of the fatal cases.
In fact the three fatal incidents in 1955 had lead me to a hypothesis which I had to verify. As the blood contains carbon monoxide without any being inhaled, if there is an undetected source of this toxic gas, it would be found in samples taken in the proximity of the respiratory organs, thus carbon monoxide would be produced in the body...

...[T]he indisputable fact [was,] that carbon monoxide did not enter the breathing passages, but that the workers bent over the metal as they cut it, and the powerful flame jet made a large area of the surface incandescent. Therefore, it was my opinion that the air, having been in contact with the incandescent metal, had become "activated". When the air was breathed in, it provoked a formation of carbon monoxide in the blood at the lungs level.
The control experiment was made with the welders themselves. They were asked to wear a sandblaster's helmet, which has an air tube at the back of the neck... allowed to hang down so that the workers breathed in the air behind them. In a short time the incidences of carbon monoxide poisoning had markedly decreased... As a result, the prevention of such accidents followed. All that was needed was to give the workman an upward current of fresh air to counteract the air rising from the incandescent metal... (Experiments made in 1964 on rabbits and on humans had shown... this endogenous reaction only takes place when the metal is heated to more than 400°C.)...
After contact with incandescent metals, nitrogen atoms heated to ~400°C in open air readily bind with oxygen atoms, forming nitric oxides that undergo resonant nuclear fission upon contact with lung tissue:
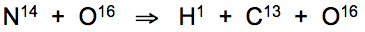
This tendency for atomic rearrangement that Kervran's experimentation had identified as occurring only above 400°C is now known to be induced by dimensional phonon resonance frequency matching of nitrogen with oxygen atoms. When heated to just above 400°C, nitrogen atoms become instilled with the resonant frequency of oxygen atoms at rest (20°C). The exact atomic data required for such complex calculations has been experimentally determined in the decades since Kervran's insightful identification.

The resonant frequency of hydrogen (H1) in its rest state is 3,773,180 Hz, according to the element's atomic diameter at 20°C. Oxygen isotope (O16) resonates at this same frequency when heated to 37.8°C:
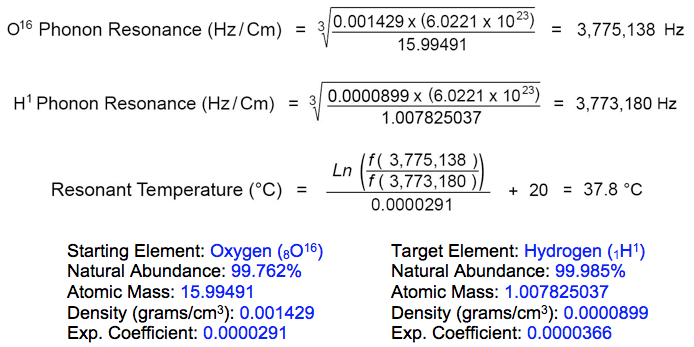
As Kervran had surmised and experimentally confirmed decades ago, potentially fatal carbon monoxide poisoning occurs as the 'atomic memory' of nitric oxides drops below 37.8°C inducing conversion of nitrogen atoms into carbon and hydrogen atoms. The low energy transmutation reaction is triggered upon contact with cooler lung tissue, without breaking the molecular bond with oxygen during the event.
Phonon resonance determinations based on the conversion formulae of W. Lussage comprehensively elucidate the mnemonic capacity and temperature-induced conversion patterns among the isotopes of the physical elements, bringing human consciousness toward a complete understanding of the fundamental atomic processes that drive the recycling of elements through the incessant electrically-enhanced interaction of metals and gases being alternately heated and cooled as the planets spin.
Copyright 2013-2017 Alexander Putney