




Resonant Transmutation of Metals in Glass Blood
Antarctic Icefish Convert Iron into Manganese at the Freezing Point
by Alex Putney for Human-Resonance.org
October 28, 2012
Many of the most extreme examples of highly specialized adaptations witnessed in animal physiology have developed in the Southern Ocean --at the freezing point of water. The intriguing metabolism of Antarctic icefish has amazed biologists for decades for their unique transparent blood variety and specialized ability to produce antifreeze glycoproteins throughout their body tissues to thrive in an icy seawater habitat that maintains regular annual temperature fluctuations between -2° and +2°C. As ocean temperatures settle below zero, icefish antifreeze glycoproteins bind and arrest the growth of ice crystals within tissues by thermal hysteresis due to greater solute concentration (Harding et al., 2003).
The role of metals in the bodies of 'glass-blooded' icefish is highly unusual, with metallothionein found to be entirely absent in most species (Scudiero et al., 1997). Hemoglobin and myoglobin, important intracellular oxygen-binding proteins, are also largely absent in many icefish species due to known gene deletions. Variable expression of the myoglobin gene also occurs by two other distinct molecular mechanisms, suggesting that loss of myoglobin expression occurred separately in at least three lineages of icefish during species diversification (Sidell et al., 1997). Subsequent investigations of the myoglobin gene in muscle tissue from the blackfin icefish Chaenocephalus aceratus identified a duplicate TATAAAA sequence that effectively blocks hemoglobin production (Small et al., 2002).

Of the 100+ distinct icefish species identified thus far, a great majority display the same set of extreme anatomical enhancements, especially enlarged hearts, with enlarged and more numerous blood vessels. The glass blood of icefish consists primarily of water, which itself carries far more dissolved oxygen near its freezing point, which is absorbed through transparent gills --and through skin surfaces.
Being scaleless, the skin of icefish acts as an integral part of the respiratory system to absorb oxygen directly from oxygen-rich Antarctic waters, effectively bypassing the oxygen-transport role of hemoglobin in the blood (as with frog skin). The newly discovered biophotonic function of blood medium is achieved in icefish by sustaining conversions of iron into hydrogen and manganese. Trace element analysis of the organs and tissues of Antarctic icefish reveal significantly elevated levels of manganese, as reported in the tissues of Champsocephalus gunnari (Ishikawa et al., 1990):
Typical accumulations were observed for Mn, Fe and Cu in muscle, whereas Zn was in the gill. Though porphyrin was absent, Fe content in the muscle of the icefish was 49.2 ppm. Also the icefish muscle accumulated a few hundred times more Mn than the other fish. The results suggest that the oxygen transporter is not the metal protein but other substances in the icefish. Mn may play an important role in specific oxygen respiration mechanisms in the Antarctic fish, Champsocephalus gunnari.

The expansive Southern Ocean is also populated by warm-blooded mammals that developed a slightly different strategy to withstand the harsh sub-zero temperatures experienced in the depths of winter. Insulation provided by a thick fat layer maintains red blood circulation between 35 - 39°C within the bodies of whales and seals, whereby resonant atomic transmutation of iron into copper is enabled by phonon frequency matching of Fe57 atoms at 37.5°C with Cu65 atoms at rest:
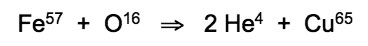
This resonant fusion reaction of iron and oxygen atoms that occurs in the hemoglobin of red-blooded mammals, and at a lower rate in the red-blooded fishes, cannot take place in icewater. Instead, a fission reaction has been effectively exploited by the near-freezing glass blood of the icefishes, which circulates much more slowly than the red blood of fish, sharks, seals and whales. Transparent blood and icefish tissue display levels of manganese that are a few hundred times greater than those of tropical fish, resulting from sustained nuclear transmutations of iron into hydrogen and manganese:
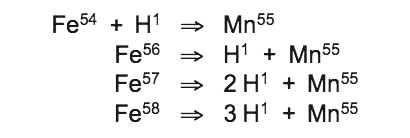

Such high concentrations of manganese in icefish cannot be explained as bioaccumulation products, but instead comprise solid evidence for perpetual resonant atomic reaction cascades releasing biophotons that are part of the essential vital processes enabling life within such harsh temperature extremes. Fission of iron atoms to form hydrogen and manganese atoms occurs at the resonant frequency of manganese target isotope (Mn55) at rest state: 43,416,442 Hz. Iron starting isotope (Fe58) resonates at this exact phonon frequency during precision cooling to 0.0°C:
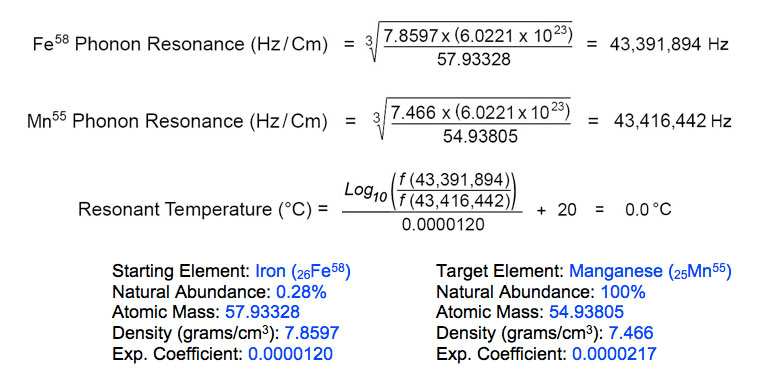
As shown, icefish metabolism can only occur at the freezing point of water. This Fe into H, Mn fission reaction was previously suggested after laboratory investigations of manganobacterial residues deposited on weathered ferruginous rocks, as well as prehistoric cave paintings made with iron-rich red ochre pigments (Kervran, 1964).
Cross-referencing the latest findings of phonon resonance physics with the unusual habitat temperatures and trace element anomalies observed of Antarctic icefish establish a more precise context for determination of the specific nuclear reactions involved. While exact dimensional phonon frequency references were not available during the speculative biophysical work of Kervran, precise phonon relationships observed among the entire periodic table of elements can now be applied to a deeper understanding of the atomic source of the vital luminosity of all living organisms, and the fundamental biophotonic function of blood medium and the DNA exciplex laser array.
Copyright 2012-2015 Alexander Putney