




Resonant Transmutation of Metals in Blue and Green Blood
Pelagic Alchemists Transmute Copper into Iron --into Titanium and Vanadium
by Alex Putney for Human-Resonance.org
October 5, 2012 - Updated November 5, 2013
The majestic beauty of all life is vitalized by the cosmic emanations of resonance. The brilliant twinkling of tremendous thermonuclear reaction cascades taking place within all stars is driven by the same fundamental nuclear transmutation processes that cause our skin to glow in the range of visible light.
The luminosity of all living matter attests to an excited electrophotonic state that can now be identified as the result of resonant nuclear transmutation cascades releasing soft photon radiation that forms standing light waves within DNA molecules. While it has been known for decades that DNA comprises an exciplex laser array displaying efficient photon storage within base molecules (Popp et al., 1984), the origin of biophotons being stored by the DNA laser array has remained entirely occluded from scientific perception --until now. Newly discovered resonant processes reveal the nuclear fire of all life.
Comprehensive findings concerning the excited state maintained by blood have elucidated the nuclear chemistry of seemingly spontaneous biophoton radiation. The known respiratory functions of iron atoms in hemoglobin to bind and transport oxygen throughout the body occurs within a finer subset of conditions that trigger atomic reaction cascades responsible for observed biophoton emission.
The primary biophotonic function in warm-blooded species requires thermoregulation to sustain dimensional phonon resonance relationships between metal nanoparticles and dissolved gases in the bloodstream that only occur within a narrow temperature band at 37.8°C. When bound with oxygen, atoms of iron isotope (Fe56) undergo fission into hydrogen and manganese atoms:
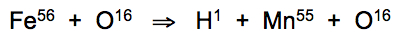
Subtle temperature fluctuations sustained by blood circulation in warm-blooded animals mirrors the greater temperature variance of ambient environmental conditions that induce similar life-sustaining resonant transmutations in cold-blooded organisms. Sunlight drives diurnal temperature fluctuations caused by the rotation of planets around their suns, which define the vibratory interactions of matter.
While land-based plant life thrives in specific habitat regions where crucial temperature resonance requirements are met, aquatic organisms undertake mass nocturnal migrations toward warmer surface waters to sustain the sensitive nuclear reaction cascades generating light within their bodies. The nightly rise of plankton and invertebrates up the water column thermocline has been explained as a feeding strategy to graze richer pelagic waters under protection of darkness, yet must be further understood as optimizing temperature-dependent phonon resonance reactions crucial to life.

The conversion of iron into copper employed by red-blooded animals is utilized in reverse as a fission reaction by blue-blooded marine animals (the cephalopods, gastropods and crustaceans) and a great variety of terrestrial and aquatic plant species (Kervran, 1964). Copper (Cu63) atoms release helium atom pairs to form manganese (Mn55) atoms, while copper (Cu65) atoms release helium atom pairs to form iron (Fe57) atoms, according to the conversion formulae:
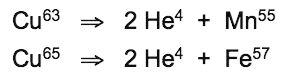
Resonant conversions of dietary copper into iron and manganese are employed by vast multitudes of lifeforms that do not regulate their own body temperatures, because the narrow temperature band required for such conversions lies very close to the mean ambient temperature in a great variety of habitats. However, careful calculation of phonon relationships reveal that frequency resonance of copper with iron and manganese cannot occur anywhere near ambient ocean temperatures.
These copper fission reactions constantly occurring in blue blood produce oxygen in the presence of hydrogen by phonon frequency matching of deuterium at 24.3°C with helium atoms at rest (20°C). The most common heavy isotope of hydrogen, deuterium is present in the rivers, lakes, seas and oceans at trace levels near 0.0156%. Aquatic organisms of all kinds inhabiting the ocean's pelagic zones exploit helium-producing phonon resonance reaction cascades by following a rising and falling band of seawater near 24.3°C, while nitrogen at ocean temperatures resonates with oxygen at rest.
Being adjacent elements on the periodic table that are most abundant in our atmosphere, nitrogen and oxygen atoms display similar structures and atomic diameters allowing phonon resonance to occur very close to the 20°C rest state. This relationship allows the phonon vibrations of hydrogen atoms absorbed into metal nanoparticles to instill the target frequency of oxygen.
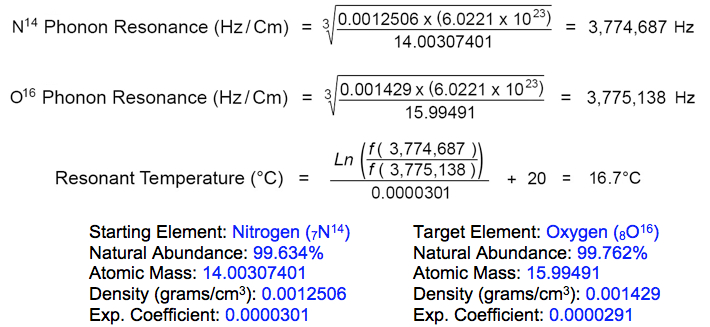
Resonant nuclear fission of copper atoms to form oxygen, manganese and iron occurs at the resonant frequency of target isotope (O16) in its rest state --at 3,775,138 Hz. Copper starting isotopes (Cu63, Cu65) become instilled with the phonon frequency of oxygen target isotope (O16) by the vibrations of absorbed nitrogen (N14) atoms within the metal lattice, during precision cooling to 16.7°C:

Similar fission reactions can be induced in iron atoms at the same resonant 16.7°C temperature, whereby terrestrial and aquatic plants are able to convert iron into calcium and potassium, as also exploited by cold-blooded animals in the growth of bone, carapace, protective shell or eggshell:
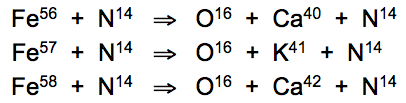
The cascade of oxygen-producing reactions at 16.7°C proceeds with an additional step following the same isotopic conversion pattern, involving transmutation of calcium into oxygen and magnesium:

The most unusual atomic conversions taking place in the animal kingdom have raised eyebrows among biophysicists for years, who have been unable to satisfactorily account for the exceedingly high concentrations of exotic metals in the bodies of sea squirts, and virtually all ascidian species. Titanium is the exotic byproduct of resonant conversions of copper into oxygen in green-blooded species that can also be induced at the calculated phonon frequency resonance threshold of 16.7°C:
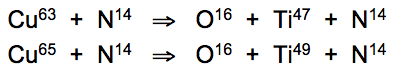

Just as blue-blooded animals utilize copper, green-blooded animals also rely on the close phonon frequency matching of nitrogen and helium atoms to induce resonant nuclear conversions of copper, iron and calcium. Trace metal analyses of the green blood of tunicates reveal high concentrations of rare metals within tunichrome blood pigments that comprise solid evidence of low energy nuclear transmutations.
The blood of Pyura chilensis contains high levels of iron and titanium while Ascidia dispar presents high levels of iron, titanium and vanadium (Roman et al., 1988). Nuclear reaction cascades in green blood involve fission of zinc atoms, induced by bound nitrogen atoms achieving phonon resonance at 16.7°C with oxygen atoms at rest, to produce oxygen, titanium, vanadium, chromium and iron atoms:
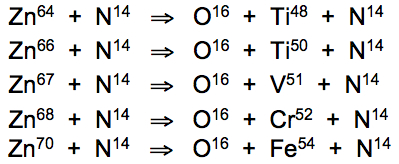
Isotopic variation of trace elements of green-blooded organisms have not yet been comprehensively studied and may reveal further reactions. In the case of human blood, perpetual Cu65 enhancement provides definitive confirmation of ongoing resonant conversions of copper into nickel and zinc. Further studies of tunicate metabolism may establish if ascidians, when deprived of an environmental source of titanium and vanadium, are capable of transmuting those elements from available copper and zinc.

These clearly observed elemental resonance patterns in the blood chemistry of all three major blood varieties in animals (red, blue and green) comprise a completely new physical framework for the development of a comprehensive understanding of the essential vital processes that perpetuate resonant nuclear recombinations releasing biophotons in all living systems.
The specific resonant temperature ranges established by basic phonon frequency determinations have been verified through extensive experimental investigations, with replication of transmutation results in both biological and non-biological systems. The great potential for the realignment of human technologies with sustainable natural resonance principles now demands our complete abandonment and dismantling of the entirely fallible atomic power facilities in use today.
Newly distinguished atomic processes revealed here deepen our mathematical knowledge of the constant regenerative abundance of nature, offering a new definition of the fundamental requirements of all life in the Universe as electrophotonically enabled by the innate, physical properties of the elements. The periodic table must be re-evaluated in new supercomputer analyses, now capable of applying resonant transmutation formulae to the revelation of all possible pathways for phonon-induced atomic transmutations throughout the entire breadth of stable elements.
We must now reshape our lives and our technologies in this rooting awareness of the creative force of resonance that operates at the core of Nature's mind, emanating the light of all life. Previously hidden to us, this light of life shines even in the deepest abyssal crevasses, springing forth at geothermal vents below our vast oceans, and those of innumerable planets throughout the depths of the cosmos.
Copyright 2012-2015 Alexander Putney