



Resonant Atomic Transmutation of Zinc into Copper
The Geyser Reactor: Zinc to Copper Transmutation
by Alex Putney for Human-Resonance.org
August 13, 2014
The resonant atomic transmutation of zinc into copper is achieved by precision control of atomic resonance in a two-stage reaction that is safe, non-toxic and involves only low energies. Zinc becomes instilled with the resonant atomic frequency signature of hydrogen, before being rapidly quenched to trigger bulk conversion into hydrogen and copper --according to the established frequency 'memory' of the standing wave field of each atom.
The first stage of the transmutation reaction maintains the starting element (zinc) at the phonon resonance frequency of the target bi-product element (H1), during a 3-hour dwell time exposed to O2 gas nanobubbles.
Protium, the lightest hydrogen isotope (H1) provides the resonant target frequency for this reaction, as determined by the following formulae (calculated using the latest atomic data sets for the elements, provided in blue):
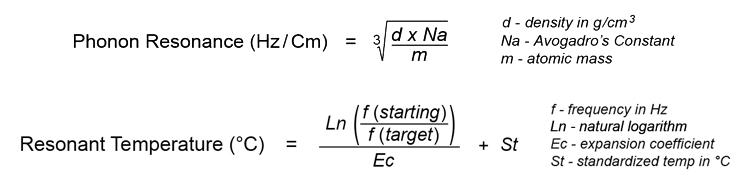
The resonant frequency of hydrogen isotope (H1) in its rest state is 3,773,180 Hz, according to the element's atomic diameter at 20°C. Oxygen isotope (O16) resonates at this same frequency when heated to 37.8°C:
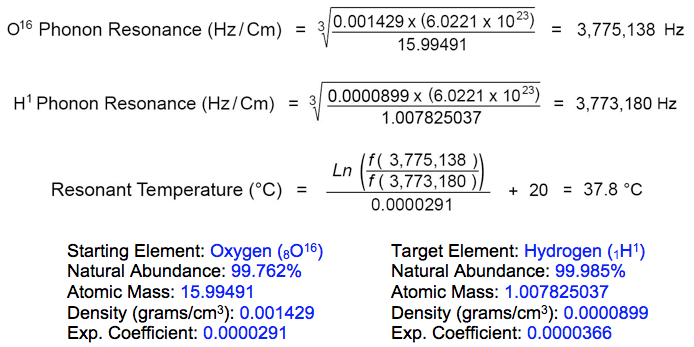
The rate of gas absorption (velocity of penetration) into the metal surface interface is enhanced by increased temperature, pressure and electric current. Oxygen gas adsorbed onto metal surfaces undergoes molecular dissociation and subsequent absorption into the crystal lattice of heavier metal atoms (above).
The second stage of the transmutation reaction involves the subsequent trapping of absorbed gases into zinc atoms by the induced shock of rapid cooling. The sudden contraction of the atomic lattice forces the interstitial absorbed atoms into quantum instability as the strongly repulsive nuclear forces of the adjacent metal atoms close in simultaneously on each gas atom from 6 sides (along the x, y and z axes of the lattice).
Instead of rapidly contracting, a portion of the zinc atoms are able to maintain the previously established resonant diameter by accepting protons, neutrons and electrons from the 6 adjacent trapped gas atoms, thereby increasing the atomic weight of zinc atoms to induce the formation of hydrogen and copper.
The face-centered cubic structure of the metal's atomic lattice allows for a maximum of 6 interstitial gas atoms being adjacent to any one metal atom, providing stable loci for gas atoms ejected by zinc atoms during quantum trapping events. Absorption of oxygen atoms through zinc surfaces at 37.8°C transmits phonon vibrations throughout the metal lattice at the hydrogen target frequency, enabling bulk conversion of zinc into copper at high rates of efficiency. The subtle bulk weight changes resulting from this resonant nuclear reaction can only be determined after ejected hydrogen atoms have been desorbed from metal surfaces by cooling to the rest state at 20°C.
Zinc to Copper
The oxygen-dependent low energy transmutation of zinc into hydrogen and copper occurs in aqueous reactors, based on phonon matching of oxygen (O16) at 37.8°C with hydrogen (H1) at rest (20°C), as in human blood.
* Zinc is heated to 37.8°C with O2, absorbing carbon and oxygen to form hydrogen and copper during rapid cooling:
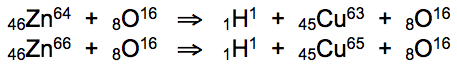
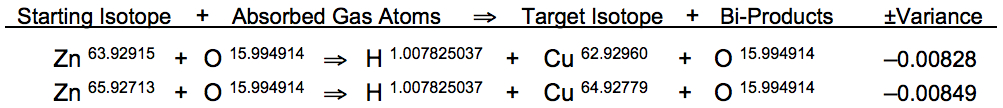
Hydrogen atoms comprise the exact atomic mass required for the conversion of zinc into copper. Clear atomic mass recombination patterns define fission bi-products ejected from zinc nuclei during the quantum trapping event:
From the book The Geyser Reactor
Return to the article The Geyser Reactor
Copyright 2013-2015 Alexander Putney