



Resonant Transmutation of Bone Calcium
Nuclear Conversions of Blood Iron into Bone Calcium
by Alex Putney for Human-Resonance.org
March 7, 2013 - Updated April 18, 2013
The primary function of blood in the formation of bone has been extensively studied, yet remains an area of vertebrate physiology that is not fully understood. Bone health has been closely linked with diet, as calcium depletion has become a major problem for westerners due to decades of regular consumption of processed milk, which has been conclusively shown to deplete calcium levels within the body rather than rebalance them as falsely advertised by the dairy industry and government agencies.
Preliminary research by C.L. Kervran provided the first coherent evidence for this counter-intuitive biological mechanism being the result of low energy nuclear transmutations taking place within the body itself, suggesting that supplementing levels of other crucial elements may achieve rebalancing of bone calcium during depletion. To this end, simple dietary trials were conducted that revealed horsetail tea effectively increases calcium levels in the body by supplying silicon for conversion into calcium.
Magnesium is another essential element implicated in the dietary rebalancing of calcium levels, but the resonant atomic conversion of one element into another could not be replicated in experiment to verify Kervran's strong hypothesis. Recent studies of isotopic variation in bone calcium versus tissue calcium reconfirms that bone biomineralization requires low energy transmutation (Skulan & DePaolo, 1999):
Calcium from bone and shell is isotopically lighter than calcium of soft tissue from the same organism and isotopically lighter than source (dietary) calcium. When measured as the 44Ca/40Ca isotopic ratio, the total range of variation observed is 5.5%, and as much as 4% variation is found in a single organism. The observed intraorganismal calcium isotopic variations and the isotopic differences between tissues and diet indicate that isotopic fractionation occurs mainly as a result of mineralization...
Bone is typically depleted in heavy calcium relative to soft tissue and dietary calcium by about 1.3-1.5% in terms of the 44Ca/40Ca ratio. This fractionation produces differences in the isotopic composition between soft tissue, bone, and dietary calcium that under many conditions can be detected with current analytical techniques. According to our model, the difference in isotopic composition between soft tissue and dietary calcium should reflect the net bone mineral loss or gain. Soft tissue is generally similar or higher in isotope 44Ca relative to dietary calcium when bone formation is dominant and lower in isotope 44Ca relative to dietary calcium when bone mineral loss is dominant. It should therefore be possible to use measurements of soft tissue and dietary 44Ca as a qualitative indicator of the calcium balance in living organisms.
Digitally-controlled phonon resonance reactors now enable replication of low energy nuclear reactions perpetually taking place during bone formation. Red blood cells supply the starting element used by the body to accomplish low energy transmutation of iron into calcium that accounts for the significant isotopic variation of mineralized bone calcium from dietary calcium and tissues.
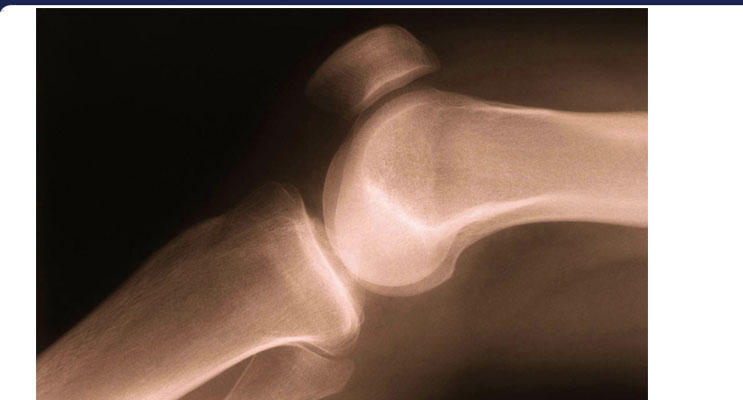
During blood circulation through the fine network of Haversian canals that course through compact bone (above), potassium atoms bound within hemoglobin transport molecules maintain rhythmic temperature fluctuations between 37-38°C that trigger the resonant conversion reaction. Each oxygen-bound atom of potassium isotope (K41) releases a single hydrogen atom during the formation of stable atoms of calcium isotope (Ca40):
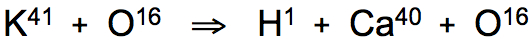
This reaction occurs simultaneously with closely related atomic conversion cascades that also include Zn into Cu, Cu into Ni, Ni into Co, Fe into Mn, Mn into Cr and Cr into V. This extensive series of reactions is facilitated within red blood cells by phonon frequency matching at the median temperature that is closely maintained within the core of the body, where the heart is located. The bones of the body are also well insulated from heat loss by muscle, fat and skin layers, comprising the core of each extremity.
Transmutation of potassium into calcium in vertebrate bone is enabled by phonon frequency matching of potassium-bound oxygen atoms with hydrogen atoms at rest state. The resonant frequency of hydrogen isotope (H1) in its rest state is 3,773,180 Hz, according to the element's atomic diameter at 20°C. Oxygen isotope (O16) resonates at this same frequency when heated to 37.8°C:
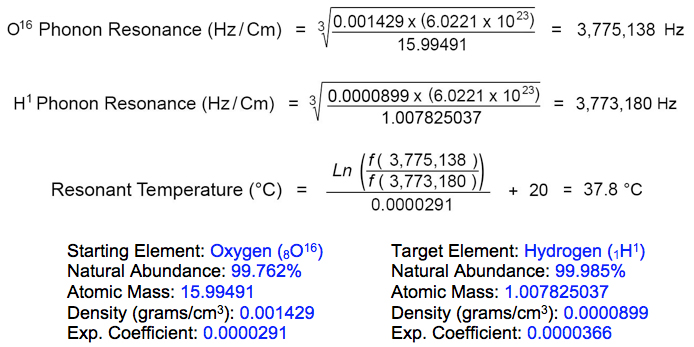
These phonon resonance determinations suggest that potassium deficiency, in particular, negatively influences the body's ability to rebalance calcium production levels, leading to gradual bone mineral loss. A balanced diet including potassium-rich foods is therefore recommended, especially white beans, dark leafy greens, baked potatoes and acorn squash, avocados, dried apricots, mushrooms and bananas, as well as herbal beverages such as horsetail tea.
For these previously unrecognized reasons, maintaining balanced, potassium-rich dietary choices effectively protects against osteoporosis by providing the elements used by the body for biomineral deposition of calcium, as specifically required for the growth and maintenance of healthy bones.
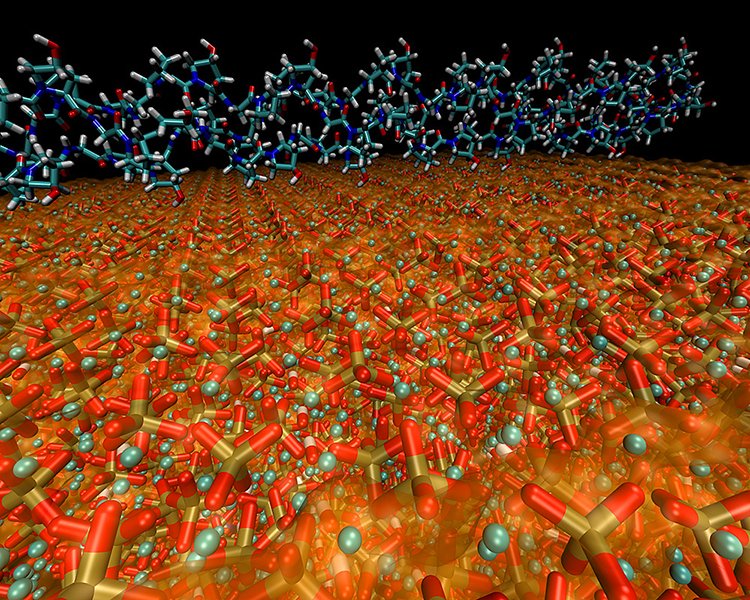
The microstructural properties of bone have been characterized in recent studies as a nanocomposite of collagen and hydroxyapatite that far exceeds the performance capabilities of both components (above, with collagen fiber atop a hydroxyapatite crystal). The complete molecular structure of bone was finally decoded by a team of MIT research scientists led by M. Buehler, after tests confirmed their calculations:
Buehler explains that even a few years ago, the modeling required to deduce the internal structure of bone would have taken years of computer time on the most powerful computers. But newer supercomputers can carry out much more detailed computations in a few months. Still, it took several iterations, after studying the previous results and probing the material's response to increasing pressure, to derive an answer, which the team was then able to confirm through comparisons with laboratory tests.
One key, they found, is that the hydroxyapatite grains are tiny, thin platelets just a few nanometers (billionths of a meter) across, and are deeply embedded in the collagen matrix. The two constituents are bound together by electrostatic interactions, which allows them to slip somewhat against each other without breaking.
This breathrough in the modeling of the molecular structures of naturally formed bone will enable development of more advanced artficial bone composite materials that will merge with natural bone without disturbing its natural piezoelectric capacity that is essential to its resonant function as a tranducer of localized infrasound. Application of supercomputer analyses to biological phonon resonance transmutation processes involved in bone formation will reveal the complete architecture of temperature-induced nuclear reactions that drive the eternal transformative rematerialization of Creation, ceaselessly turning the atomic wheelworks of Nature.
Copyright 2013-2017 Alexander Putney