




Resonant Atomic Transmutation of Silver into Gold
The Geyser Reactor: Silver to Gold Transmutation
by Alex Putney for Human-Resonance.org
August 13, 2014
The resonant atomic transmutation of silver into gold is achieved by precision control of atomic resonance in a two-stage reaction that is safe, non-toxic and involves only low energies. Silver becomes instilled with the resonant atomic frequency signature of gold, before being rapidly quenched to trigger bulk conversion into gold --according to the established frequency 'memory' of the standing wave field of each atom (rendered below).

The first stage of the transmutation reaction maintains the starting element (silver) at the phonon resonance frequency of the target element (gold), during a 3-hour dwell time exposed to CO2 gas nanobubbles.
Gold's single isotope provides the resonant target frequency, as determined by the following formulae (calculated using the latest atomic data sets for the starting element and the target element, provided in blue):
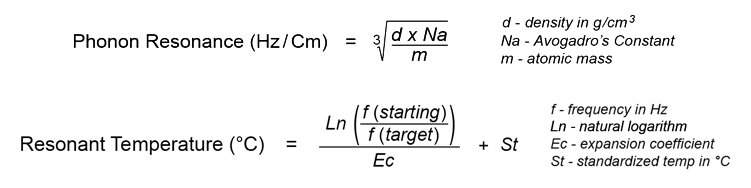
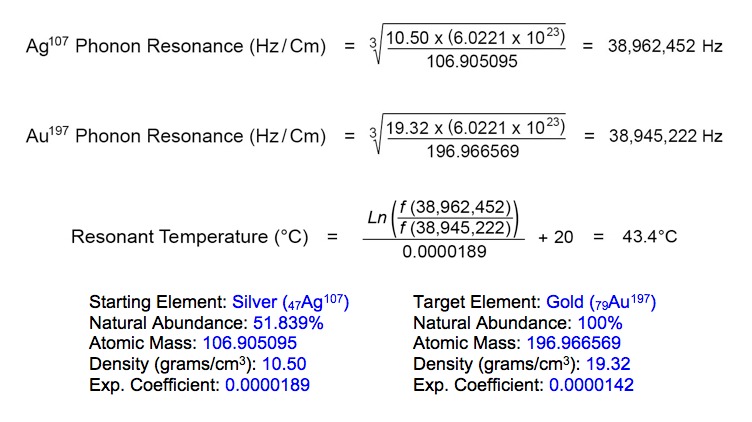
The resonant frequency of gold (Au197) in its rest state is 38,945,222 Hz, according to the element's atomic diameter at 20°C. The lighter isotope of silver (Ag107) resonates at this same frequency when heated to 43.4°C:
The rate of gas absorption (velocity of penetration) into the metal surface interface is enhanced by increased temperature, pressure and electric current. Carbon dioxide gas adsorbed onto metal surfaces undergoes molecular dissociation and subsequent absorption into the crystal lattice of heavier metal atoms (above).
The second stage of the transmutation reaction involves the subsequent trapping of absorbed gases into silver atoms by the induced shock of rapid cooling. The sudden contraction of the atomic lattice forces the interstitial absorbed atoms into quantum instability as the strongly repulsive nuclear forces of the adjacent metal atoms close in simultaneously on each gas atom from 6 sides (along the x, y and z axes of the lattice).
Instead of rapidly contracting, a portion of the silver atoms are able to maintain the previously established resonant diameter by accepting protons, neutrons and electrons from the 6 adjacent trapped gas atoms, thereby increasing the atomic weight of silver atoms to induce the formation of gold atoms.
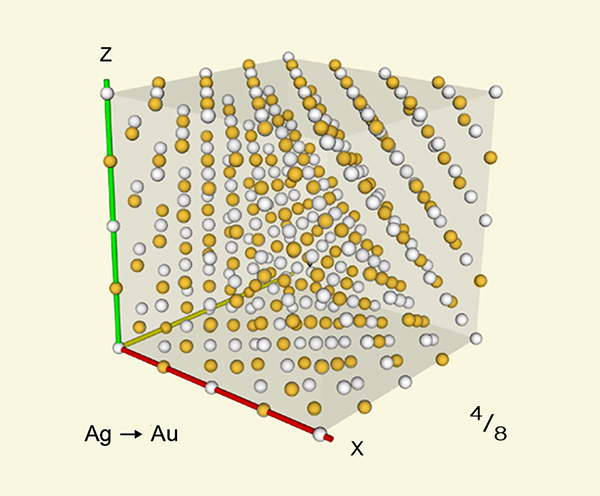
The face-centered cubic structure of the metal's atomic lattice allows for a maximum of 6 interstitial gas atoms being adjacent to any one metal atom, limiting maximum total conversion during rapid cooling to below 1/8th (~12.5%) of the total number of metal atoms. For optimal cases with complete saturation of absorbed carbon dioxide gas (illustrated below), transmutation during a single rapid cooling event can convert upto 12.5% of silver atoms into gold, leaving the remaining 87.5% unaltered --thereby increasing to 110.25% of the total original mass.
Gas absorption periods of prolonged dwell-time at phonon resonance with the gold target element are interrupted by controlled rapid cooling events that accomplish the quantum trapping of absorbed gas atoms within the contracting metal lattice. This cyclical process is repeated dozens of times over each 2-week production run, with successive contractile phases gradually converting greater than 70% of silver atoms locked within the lattice.
These emerging resonant nuclear recombination dynamics dictate that no adjacent pair of silver atoms may be simultaneously converted into gold due to the limited availability of interstitial gas atoms. Experimentation with successive gas absorption and quenching phases has confirmed that >90% conversion of silver atoms into gold atoms can be achieved after a few dozen repetitions of the same basic phonon resonance process when using aqueous silver nanopowder suspensions with carbon dioxide nanobubble systems.
Silver to Gold
The carbon dioxide-dependent low energy transmutation of silver atoms into gold and hydrogen atoms occurs after prolonged absorption of carbon dioxide during precision heating to 43.4°C phonon frequency resonance with gold.
* Silver is heated to 43.4°C with CO2, absorbing carbon and oxygen atoms to form gold and hydrogen during rapid cooling:
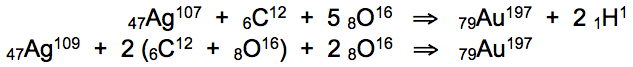
Carbon dioxide (CO2) gas comprises the exact atomic mass required for the conversion of silver into gold, after dissociated carbon and oxygen atoms are fully absorbed into silver nanoparticles. Carbon and oxygen atoms absorbed within the framework of the silver atomic lattice may recombine with individual silver atoms in groups of 6 adjacent gas atoms occupying all available interstitial loci, recombining as (1 C + 5 O) and (2 C + 4 O):
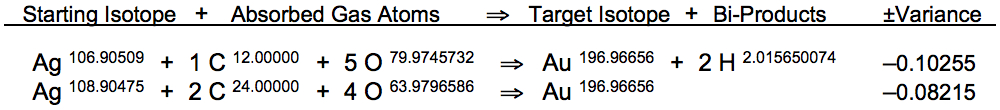




From the book The Geyser Reactor
Continue with Gold to Platinum Transmutation
Return to the article The Geyser Reactor
Copyright 2013-2015 Alexander Putney